The RAPID-WATER-FLOW Trial is a Phase III clinical research trial which uses new technology in conjunction with PET imaging to visualize and quantify blood flow in the heart muscle. The trial is sponsored by MedTrace Pharma.
The RAPID-WATER-FLOW Trial is a Phase III clinical trial to determine the efficacy of radiolabeled water to detect region(s) of narrowed or clogged blood vessels to diagnose coronary artery disease (CAD).
The study aims to determine the sensitivity and specificity of 15O-H2O (15O-water) positron emission tomography (PET) myocardial perfusion imaging (MPI) in detecting coronary artery disease (CAD), using the gold standard of invasive coronary angiography (ICA), ICA with fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR), or coronary computed tomography angiography (CCTA).
In the study, the radioactive tracer 15O-H2O is administered during the cardiac PET scans. The uptake and release of 15O-H2O by myocardial cells are directly proportional to blood flow, a property that is expected to result in higher diagnostic accuracy for ischemic heart disease compared to other PET tracers. Furthermore, 15O-H2O offers lower radiation exposure than other tracers currently used in cardiac PET imaging.
Subjects can be included in the study through one of the following pathways.
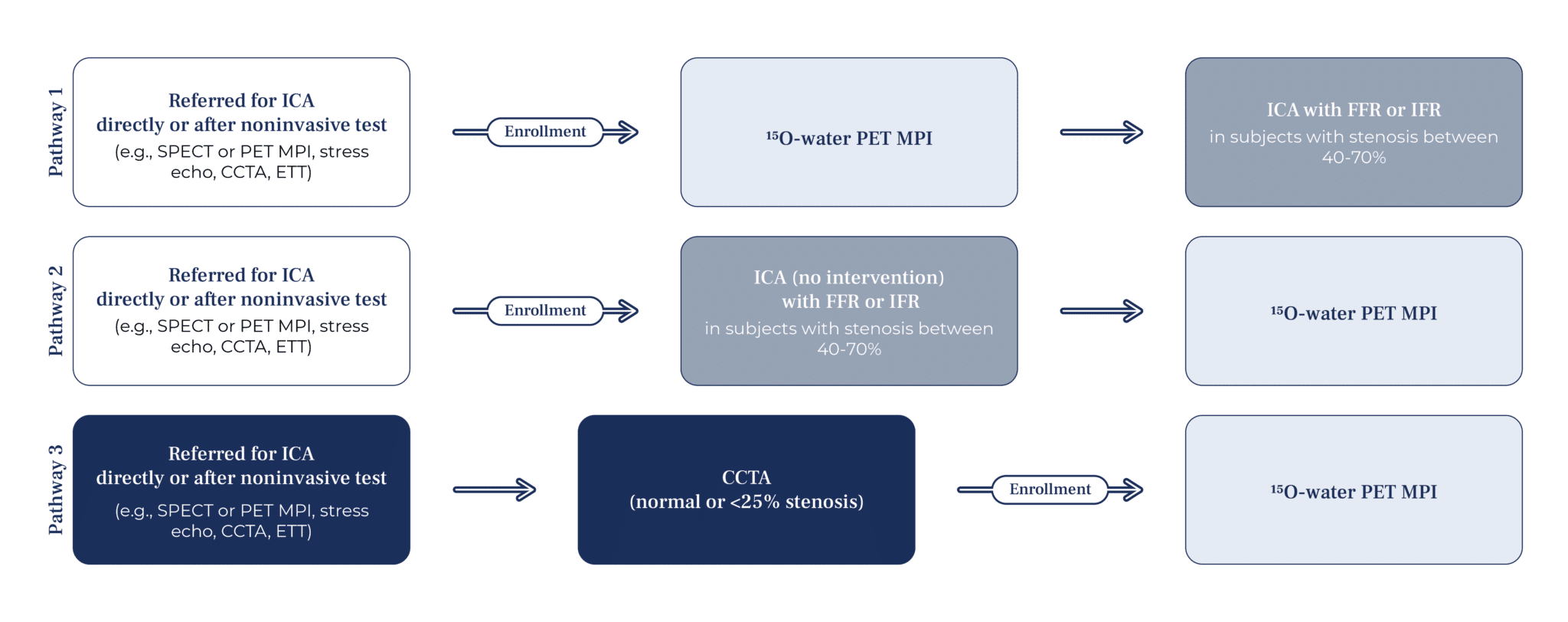
Pathway 1:
Individuals referred for invasive coronary angiography (CAG), either directly due to symptoms and risk factors or following a non-invasive test of cardiac blood flow (e.g., myocardial scintigraphy, cardiac PET, stress echocardiography, CCTA, or exercise ECG), will undergo the trial protocol with 15O-H2O cardiac PET before the invasive CAG is performed
Pathway 2:
Individuals who have already undergone invasive CAG without the need for intervention may be included in the study if any stenoses were assessed between 40% and 70%, provided that FFR or iFR measurements are available. These participants will then undergo the trial protocol with 15O-H2O cardiac PET after the invasive CAG.
However, subject with a known history of cardiac disease cannot be included in the study. (Refer to the protocol for more information.)
Pathway 3:
Individuals who have already undergone a CCTA for any reason and whose coronary arteries were found normal or minimally abnormal (<25% stenosis). These participants will undergo the trial protocol with 15O-H2O cardiac PET after the CCTA is performed.
The subject can be screened either prior to the procedure or on the same day as the scan. All participants will be included once deemed eligible and then receive two injections of the radioactive tracer during the 15O-H2O cardiac PET scan—one at rest and one during pharmacological stress induced by adenosine.
Following the scans, the study team will contact the participant by phone 24 ± 8 hours after the cardiac PET scan to follow up on any potential side effects.
The risk of acute side effects from the experimental 15O-H2O PET scan is considered minimal based on prior studies. MOST COMMON SIDE EFFECTS ARE ASSOCIATED WITH ADENOSINE. In the long term, the risk is linked to the use of ionizing radiation, which totals approximately 1.3 mSv. This places the study in the lower range of category IIb (1-10 mSv).
Ischemic heart disease (IHD) and coronary artery disease (CAD) are among the leading causes of morbidity and mortality in the Western world. Over the years, significant resources have been allocated to both the treatment and early diagnosis of IHD and CAD. Symptomatic CAD is currently treated either invasively with coronary angiography (CAG) and potential revascularization through stent placement (PCI), or medically with various anti-anginal drugs. However, invasive CAG with PCI carries a notable risk for the patient, making it essential to identify those who would benefit more from CAG rather than optimized medical management. To facilitate this, non-invasive imaging tests such as CCTA, SPECT, stress echocardiography, or PET with various tracers. Studies have shown that 15O-H2O PET offers a higher accuracy in detecting CAD, while also exposing patients to approximately 50% less radiation.

Stay connected with MedTrace and the RAPID-WATER-FLOW Study through our newsletter by subscribing today.

E-mail: laurel@medtracepharma.com
Phone: +1 773 330 2202

We use technologies such as cookies to measure traffic and optimize the website. By clicking ‘Accept’ you consent to these technologies. Failure to consent may have a negative impact on certain features.